Key points
- Implant reconstruction involves reconstructing the breast using a silicone breast implant.
- The surgery is performed as a one-stage or two-stage procedure and will often involve multiple follow up appointments for expansion.
- Often recommended for women with smaller breasts.
- Complications include risk of rupture or leakage of implant and asymmetrical breasts.
- Replacement of implant
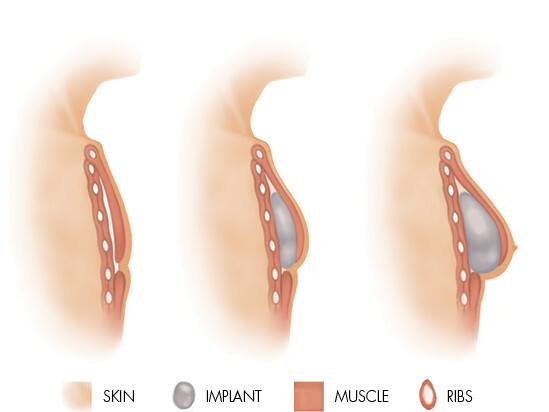
Implant reconstruction
Implant reconstruction involves inserting a silicone breast implant under the skin and pectoral muscle (or, less often, over the muscle) post-mastectomy. The implant is supported by a dermal matrix, which eventually becomes part of the structure of the reconstructed breast.
Implant reconstruction is often recommended for women who have smaller breasts, and who want to avoid either a long, complex operation and/or donor scars. Recovery time is reduced following implant reconstruction.
Implant reconstruction techniques
One-stage procedure
Insertion of a permanent implant at the time of mastectomy is possible if sufficient skin has been preserved, leaving an adequate envelope of skin and muscle to cover the implant.
Two-stage procedure
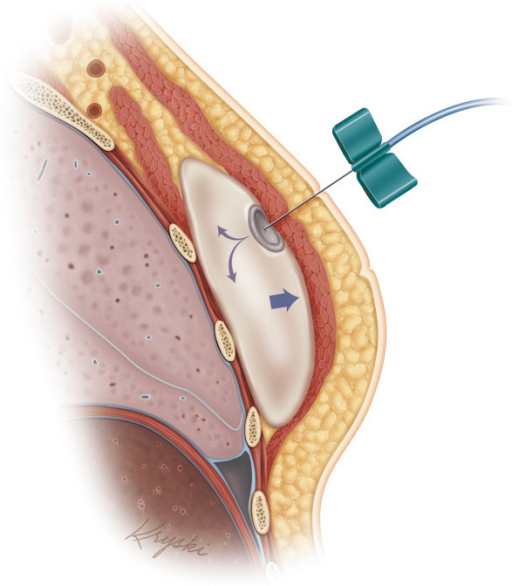
A tissue expander is inserted during the mastectomy and partially filled with saline. Once the area has healed, more saline is injected over several weeks to gradually increase the expander volume and stretch the muscle and skin, to eventually create a large enough pocket for a permanent implant.
Typically, the expander is ‘over-expanded’ to ultimately improve the shape of the reconstructed breast and provide symmetry to the remaining breast. Once filled, the expander is left in the breast for several months to let the skin adjust. A second operation replaces the expander with a silicone or saline gel implant. Surgery to the remaining breast (e.g. for symmetry) is then performed.
Factors to consider
- Is recommended for women who are small in stature or don’t have the tissue required for an autologous reconstruction.
- Operation time is shorter
- May be recommended for complications such as, diabetes or obesity that can increased risk of delayed wound healing and infection.
- Expansion process may require multiple follow up appointments.
- The implant may eventually need to be replaced.
- Timing of surgery is important if radiation is part of the treatment plan.
- Tissue expansion may be limited in women who have had prior radiation therapy.
- Risk of rupture or leakage of implant.
- Risk of capsular contracture, requiring removal of the implant in some patients.
- Risk of breast implant-associated anaplastic large cell lymphoma is an extremely rare side effect and treated by the removal of the implant and surrounding capsule.
- Implant volume is fixed, therefore any changes to the remaining breast due to age or weight fluctuations will cause breast asymmetry.
- Monitoring the condition and placement of the implant may require regular ultrasound